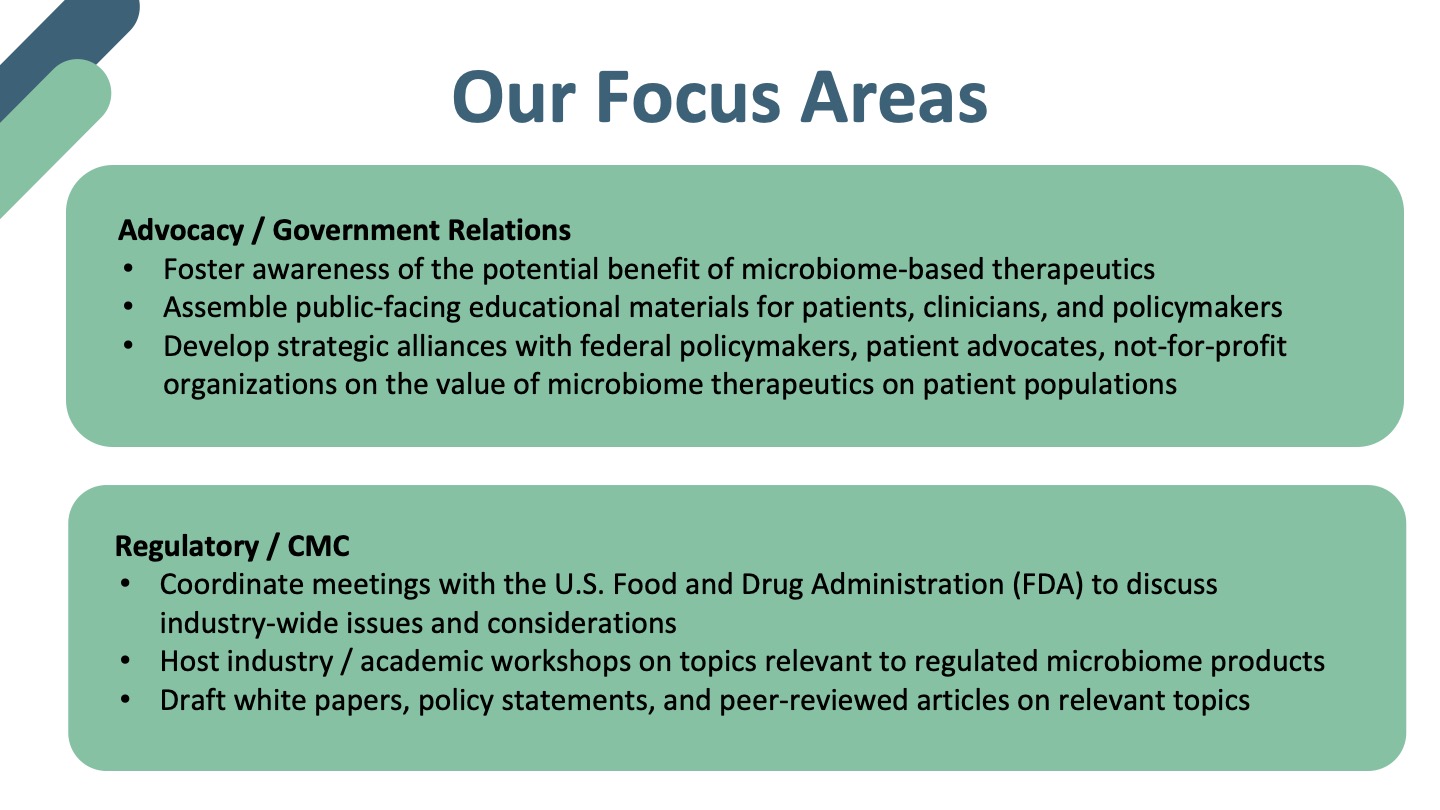
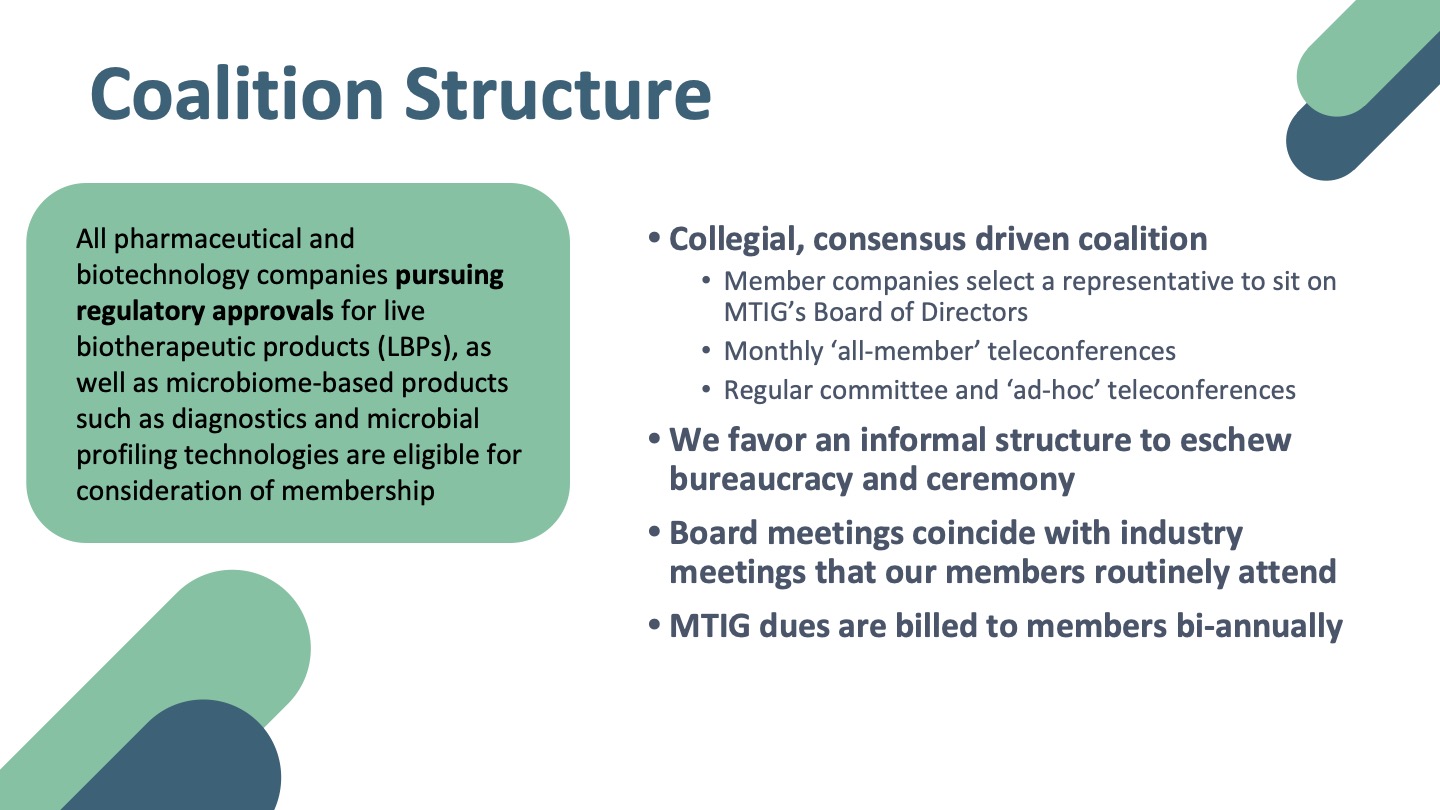
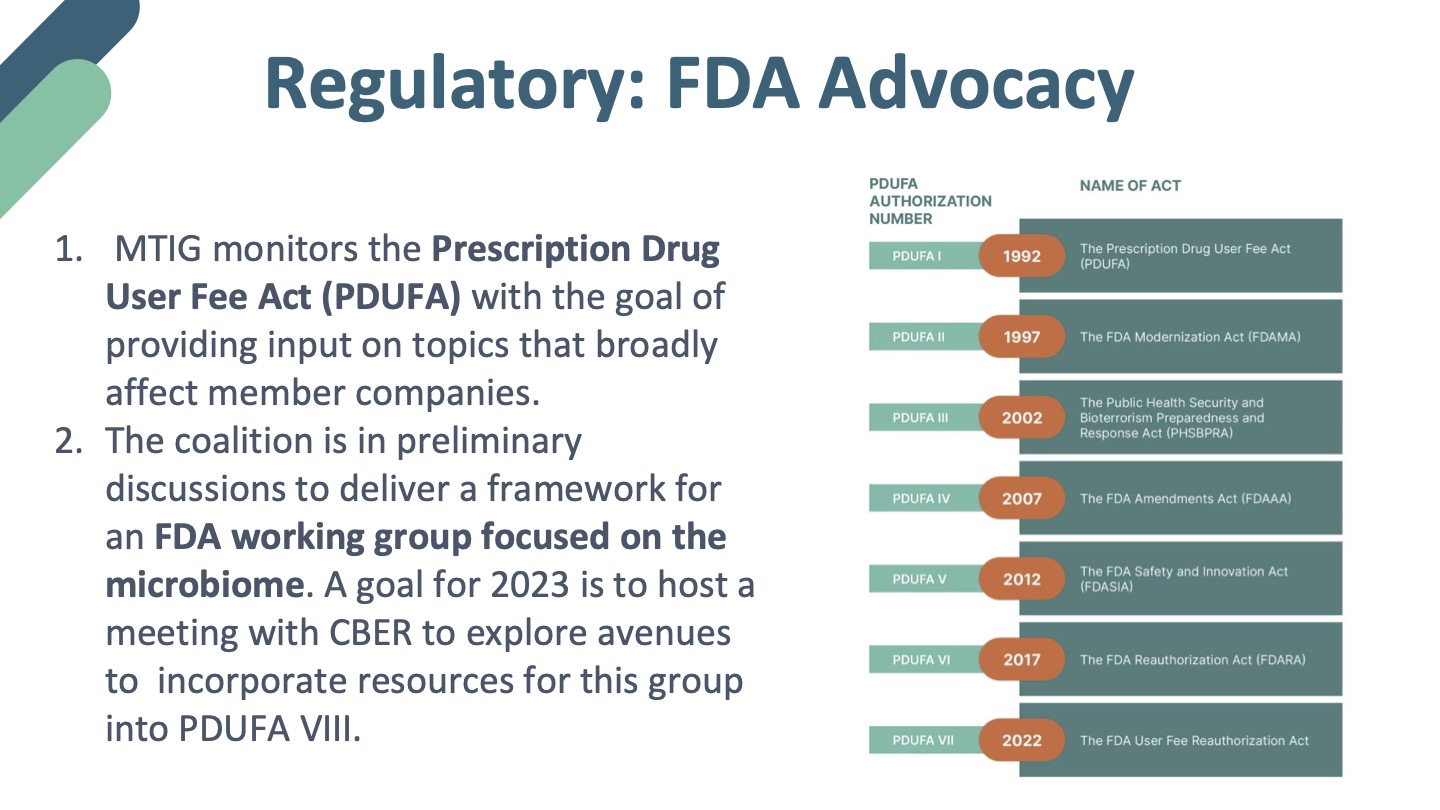
About the MTIG
The MTIG Mission
The Microbiome Therapeutics Innovation Group (MTIG) is an independent 501(c)(6) coalition of companies leading the research and development of FDA-approved microbiome therapeutics and microbiome-based products to address unmet medical needs, improve clinical outcomes, and reduce health care costs. The human microbiome is one of the new frontiers of medical innovation that has the potential to benefit patients suffering from numerous diseases afflicting millions of patients and consuming billions of dollars of healthcare resources. Through a collective voice, the MTIG membership works together to enhance the regulatory, investment, and commercial environment to accelerate microbiome therapeutic product development and expand availability of life-changing and life-saving FDA approved microbiome therapies to patients. MTIG is committed to working with stakeholders who share in our mission and seek to collaborate with patients, patient advocates, professional societies/organizations/public health officials, scientists, government policymakers, healthcare providers and payers who seek tangible policy and regulatory solutions to issues in the emerging microbiome arena.
Board of Directors

Nikole Kimes, Ph.D
Chair of the MTIG Board
Founder & Chief Executive Officer
Siolta Therapeutics
As a founder and the lead executive driving Siolta’s early-stage development, Nikole heads a talented team of scientists, blending microbiology, immunology, and bioinformatics expertise to leverage microbiome data for the improvement of patient stratification and development of precision microbial therapeutics. Nikole has over a decade of experience in microbial ecology and host/microbe interactions, supported by the National Science Foundation (NSF), the European Commission FP7, and the University of California San Francisco (UCSF). As an inventor of Siolta’s technology, her research in Dr. Susan Lynch’s lab at UCSF provided the foundation for the company’s translational research program.

Ken Blount, Ph.D
Chief Scientific Officer
Rebiotix Inc.
Ken Blount is the Chief Scientific Officer of Rebiotix, a results-oriented biotechnology company revolutionizing the treatment of challenging gastrointestinal diseases by harnessing the power of the human microbiome.
Dr. Blount brings 20 years of experience leading disruptive drug discovery and development programs.. He has initiated, directed, and grown collaborative research programs spanning the biotechnology and pharmaceutical industries as well as research and academic institutions, and he currently serves on the Joint Steering Committee for the Center for Translational Microbiome Research at the Karolinska Institutet in Stockholm, Sweden. Prior to joining Rebiotix, he coordinated translational oncology research for the Yale Cancer Center and cofounded and directed BioRelix, a venture-backed startup that discovered and developed novel RNA-targeting antibiotics.
Dr. Blount holds a Ph.D. degree in biochemistry from the University of Colorado and a B.S. degree in biochemistry from the University of Arkansas.

Matthew Henn, Ph.D.
Executive Vice President, Chief Scientific Officer
Seres Therapeutics
Matthew Henn is Executive Vice President and Chief Scientific Officer at Seres Therapeutics. He contributed to the launch of Seres Therapeutics in 2012 and has been involved in the discovery and clinical development of multiple microbiome therapeutics including SER-109, SER-155, SER-262, SER-287, and SER-401. Dr. Henn has over 20 years of research experience focused on microbial populations and the functional role of microbes in both environmental and human disease applications, as well as the development of genomic and functional tools to study these populations. He has authored over 60 peer-reviewed publications and currently serves on the scientific advisory board of Growcentia, Inc, an agricultural microbiome company. Prior to Seres, he was the Director of Viral Genomics and Assistant Director of the Genome Sequencing Center for Infectious Diseases at the Broad Institute of MIT and Harvard. Dr. Henn earned his Ph.D. in ecosystem sciences from the University of California at Berkeley, where he was a NASA Earth Systems Sciences Fellow, and trained as a NSF Postdoctoral Fellow in Microbiology at Duke University.

Ronny Hermansen, MsBA
CEO, Genetic Analysis (GA)
Mr Hermansen has gained more than 25 years of experience from the international diagnostics industry, previously as Group CFO in Axis-Shield plc (LSE) where he worked out of London since 2007 until it was acquired by Alere Inc in Dec 2011.
Mr. Hermansen has extensive experience from management and Board positions within the diagnostics industry, including extensive acquisitions and merger work.
Mr Hermansen has been involved with Genetic Analysis since April 2014, and he holds an MsBA (Cand. Merc) from the University in Aalborg, Denmark. Previous experience includes Nycomed Imaging, Nycomed Amersham (later GE Healthcare) as Vice President of Finance in Operations.

Bernat Olle, Ph.D
Co-founder and Chief Executive Officer
Vedanta Biosciences
Dr. Olle is a co-founder and Chief Executive Officer of Vedanta Biosciences. He has been a member of the founding teams of several companies of the PureTech portfolio and served as a member of the Board of Directors of Vedanta Biosciences and Follica Biosciences.
In 2013 Dr. Olle was named “Innovator of the Year” in MIT Technology Review Spain’s “Innovators under 35” awards. He completed his doctoral work at the Chemical Engineering Department at MIT, where he developed a novel method for large-scale bacterial culture. During his graduate work, Dr. Olle was awarded the “la Caixa” fellowship. Dr. Olle received his B.S. in Chemical Engineering from Universitat Rovira i Virgili, in the Republic of Catalonia, his M.S. and PhD. in Chemical Engineering Practice from MIT, and his M.B.A. from the MIT Sloan School of Management. He has published his work in journals including Nature and Nature Biotechnology.

Blake Wills
Chief Executive Officer
Microba Life Sciences
Mr Wills is a proven company executive with experience in the finance, life sciences and education sectors. Previously the Chief Operating Officer of an ASX-listed company, Mr Wills brings substantial operational, financial and governance experience to Microba as founding CEO. His financial background combined with his extensive operational expertise has driven Microba forward as a thriving company with more than 40 full-time employees. Mr Wills’ experience in executing multiple international acquisitions and the management of new product and service builds has given him the skills to provide Microba’s world-leading technology to people globally. He is well-educated with qualifications from one of Australia’s top business Universities, with a Master of Business (Professional Accounting), Bachelor of Finance (Hons) and Bachelor of Business (Finance) from the Queensland University of Technology.

Philippe Moyen
Chief Operating Officer
Maat Pharma
The former Vice-President of business operations for PTC Therapeutics Inc., Philippe Moyen brings more than 20 years’ experience in the pharmaceutical and biotech industry, including proven operational excellence and business acumen in small molecules and Biologics that will enable him to effectively contribute to the Company’s future milestones.

Kevin Honaker
Co-Founder & Chief Executive Officer
BiomeSense
Kevin Honaker is the cofounder and CEO of BiomeSense. He is a skilled life sciences commercialization leader with extensive experience across the entire health care ecosystem, including genomic precision medicine, drug development, and provider and payor strategy. This experience led him to cofound BiomeSense to help accelerate microbiome precision medicine to widespread clinical practice. As CEO, Kevin is responsible for all strategic and operational leadership, guiding BiomeSense’s growth from a whiteboard sketch through the first clinical trials of their proprietary GutLabTM technology.
Kevin holds an MBA from the University of Chicago Booth and is a named inventor on several patents.

Johan E.T. van Hylckama Vlieg
Co-Founder & CSO
Freya Biosciences
Johan E.T. van Hylckama Vlieg is cofounder and CSO at Freya Biosciences, a series A-financed, clinical stage company developing microbial immunotherapies in women’s health. He is an experienced R&D leader and executive with over 25 years of experience in building R&D programs and organizations in public and privately-held biotech companies in both the US and Europe. His expertise spans microbe-host interactions, live biotherapeutics & microbial immunotherapies and he is (co)author on more than 75 peer-reviewed publications and inventor on more than 15 patent applications. He has built and lead science & innovation teams covering compound synthesis and strain selection, preclinical screening & development, and first-in-human testing. In addition to his scientific expertise, he brings C-level experience in manufacturing, fundraising & commercial development.
Coalition Management

Paul Kim
MTIG Regulatory Counsel
Partner
Kendall Square Strategies
Regarded as one of the top food and drug lawyers in Washington, Paul Kim draws on his extensive governmental experience to advise clients on legal, legislative and regulatory issues in food, drug and device law, Medicare and Medicaid coverage and reimbursement, and the conduct of clinical research. He represents leading biotechnology, pharmaceutical and medical device companies before the Food and Drug Administration (FDA), the Centers for Medicare and Medicaid Services (CMS), and Congress.
Paul uses his Capitol Hill experience in the enactment, amendment and implementation of the Food, Drug, and Cosmetic Act, and such landmark legislation as the Public Health Security and Bioterrorism Act, the Hatch-Waxman Amendments and the Orphan Drug Act to advise clients on compliance issues and regulatory approvals. He also assists with general FDA regulatory matters, collaborating with clients to develop comments, petitions, presentations and regulatory submissions for agency rulemakings, advisory committee proceedings, early collaboration and pre-submission meetings, and other meetings with key Federal decision-makers. Clinical research compliance, FDA product approval, CMS coverage and reimbursement issues, and bioresearch monitoring are additional areas of focus in Paul’s regulatory practice.
Paul also develops effective legislative and media strategies for industry clients, manages advocacy coalitions, and helps clients develop constructive relationships with key federal regulators and congressional decision-makers. He also represents clients before congressional committees and within their dealings with individual members of the United States Senate and the House of Representatives, defends their interests in congressional oversight and investigations, and helps them prepare for hearings.
Before joining Foley Hoag Paul was Deputy Staff Director for health policy for Senator Edward M. Kennedy, who chaired the U.S. Senate Health, Education, Labor and Pensions Committee. Major laws enacted while Paul served with Senator Kennedy include The Public Health Security and Bioterrorism Response Act of 2001 (whose food safety protections were deemed “the most significant expansion of federal authority over the food industry in more than six decades” by the New York Times), the Reauthorization of the Prescription Drug User Fee Act, The Medical Device User Fee and Modernization Act of 2002, The Rare Diseases Act of 2002 (amending the Orphan Drug Act), The Best Pharmaceuticals for Children Act, and Senate passage of the Greater Access to Affordable Pharmaceuticals Act of 2001. Paul also was Counsel to Congressman Henry A. Waxman (serving as staff in both the House of Representatives and the Senate, as well as in conference, on the Food and Drug Administration Modernization Act of 1997) and as a professional staff member to Senator David Pryor on the U.S. Senate Special Committee on Aging and the U.S. Senate Finance Committee. His other public policy experience includes positions as Assistant Director for Government Relations with the American Foundation for AIDS Research (amFAR), the largest private funder of HIV/AIDS research, as a Policy Analyst with the Office of the Commissioner, Food and Drug Administration, and with Ciba-Geigy Pharmaceuticals in Basle, Switzerland.

Stephen Conafay
MTIG Washington Counsel
Principal
The Conafay Group
Steve Conafay has 35 years of leadership and management experience in government relations, public policy and communications. As the sole principal at The Conafay Group, he oversees all client servicing. While leader of the bio-life science practice at Fabiani & Company, he oversaw more than $810 million in non-dilutive financing awarded to the team’s clients. For 12 years, Mr. Conafay was vice president of government relations for Pfizer, where he was elected as a corporate officer in 1984. He served as senior vice president of corporate affairs for Glaxo Inc., where he was also a member of the Board of Directors. After heading the legislative practice for Jones, Day, Reavis and Pogue, the world’s second largest law firm, he was tapped by the Pharmaceutical Research and Manufacturers of America (PhRMA) to assume the No. 2 position of executive vice president of strategic and legislative affairs. He also served as an executive fellow at the American Enterprise Institute, the leading free market think tank in the U.S. After moving to the New York area, Mr. Conafay joined Shandwick, where he assumed several positions of increasing responsibility, culminating with his appointment as chief executive officer for The Americas-East in 2000.
Mr. Conafay is a lawyer and a decorated Vietnam veteran. He served as an infantry platoon leader and was awarded the Silver Star, Bronze Star with Oak Leaf Cluster, the Air Medal and the Army Commendation Medal, among others. He is also an avid golfer and has been a member of Congressional Country Club for more than 30 years. Mr. Conafay is a lifelong fan of the New York and San Francisco Giants. In his 60s, he ran and completed four Army 10-miler races, and plans to continue competing in his 70s.